Life Science
Pharmaceutical industry, in-vitro diagnostics, bioprocess technology, analytics or medical technology: Every industry has its requirements, work processes and special disciplines for which you as a device manufacturer, system integrator or laboratory operator are an absolute expert. As soon as you want to digitize and automate your workflows or offer your laboratory and medical equipment a decisive user added value via the software, we are your ace up the sleeve. We support you from software prototyping in device pre-development through digital work processes in the laboratory to standard-compliant software as a stand-alone application or as a component of certified medical and laboratory products.
We are one of the few software service providers in Europe to have a certified quality management system in accordance with ISO 13485 and our own development process that meets the relevant requirements of IEC 62304 and other relevant standards. MDR-, IVDR-, FDA-, NMPA- and GMP-compliant software can therefore be developed flexibly according to your or our processes and we can provide you with comprehensive and professional support from the idea to certification. Read more ...

E-Mail:
s.buettner@infoteam.de
Phone:
+49 9131 78 00-280
I am looking for the right contact in our company for your topic and look forward to hearing from you.
Make an appointment online
Embedded software
The development of embedded software has been one of our great strengths for many years. Our software sets your hardware in motion (also with regard to real-time requirements) and creates the basis for continuous communication and networked systems – of course always with regard to current security requirements. Especially for the needs and specifications in laboratory automation and medical technology, we combine our embedded knowledge with our expertise in standard-compliant software development for medical and laboratory devices up to the highest risk class. Read more ...
Application software
Desktop applications as well as mobile apps and cloud solutions, which are becoming increasingly important in medical and laboratory technology, are our daily business. They all need to provide the highest levels of stability, security and performance while being intuitive to use, and therefore require the use of robust and future-proof technologies in addition to industry experience. Our more than 300 employees master the extensive and fast-growing spectrum of modern software development, which, together with our expertise in standard-compliant software development for medical and laboratory products, is also perfectly suited for applications in the life sciences sector.
As an experienced partner, we support you throughout the entire software and product life cycle especially in the development of standards-compliant and certified medical apps. Read more ...
Middleware
For us, standardized communication interfaces are the prerequisite for continuous networking in the laboratory and medical environment. Only in this way can devices, processes and applications be controlled decentrally and data exchanged, processed and analyzed across locations.
- For consistent data management in higher laboratory systems (e.g. LIMS) and standardized control mechanisms, we not only rely on the use of international standards such as LADS (German only) based on OPC UA, SiLA, and AnIML, but are also actively involved in the respective committees.
- For medical image data management, we use the HL7 (Health Level 7) standard, which is widely used in hospital information systems (HIS), through the use of DICOM. But we are also familiar with FHIR and other standards.
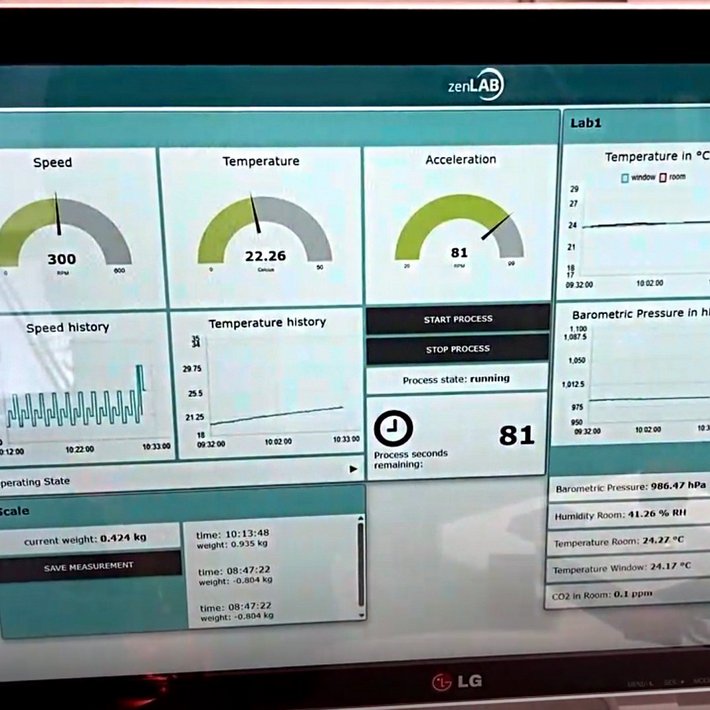
Our scalable and modular zenLAB® Middleware Framework, which is aimed at laboratory operators and device manufacturers alike, is designed specifically for the processes and requirements of the modern laboratory. zenLAB® simplifies the everyday working practices of your customers and employees by networking laboratory devices, establishing a uniform database from the laboratory to management level for the first time, and increasing the degree of digitisation from experiment execution to documentation. Unlike conventional standard software, zenLAB® does not leave you with procedures that are almost impossible to implement in your daily life. The lab-specific modules exactly mirror the requirements and processes that you, your employees and your customers need to be as efficient as possible.