Bubenreuth, February 3rd 2021
- Refined PCR method: PCA (Pulse Controlled Amplification) provides test results of up to eight samples within 20 minutes
- Special approval of the Bundesinstituts für Arzneimittel und Medizinprodukte (BfArM; Federal Institute for Drugs and Medical Devices) since 23.12.2020
- infoteam Software Group significantly developed the system software from April 2020 in Bubenreuth and Dortmund
Since April 2020, the infoteam Software Group has developed the system software for the PCR rapid test device "GNA Octea" on behalf of GNA Biosolutions GmbH from Martinsried near Munich in a case of urgency. On 23.12.2020 the test method received the special approval of the Bundesinstituts für Arzneimittel und Medizinprodukte (BfArM; Federal Institute for Drugs and Medical Devices) on December 23, 2020. GNA plans to start the special approval procedure for the EU region shortly.
Since April 2020, infoteam as a certified service provider for software development in the medical and laboratory environment spontaneously and unbureaucratically supported the development work for the system software of the mobile rapid test device. At peak times, up to six infoteam software specialists for medical and laboratory technology were withdrawn at short notice from ongoing projects in Bubenreuth and Dortmund with the support of the affected customers in order to give the development a powerful boost. “We see it as our social duty to canalize and make available our expertise in such a situation," says Alexander Brendel, Director Life Science at infoteam Software AG.
Key data for GNA Octea
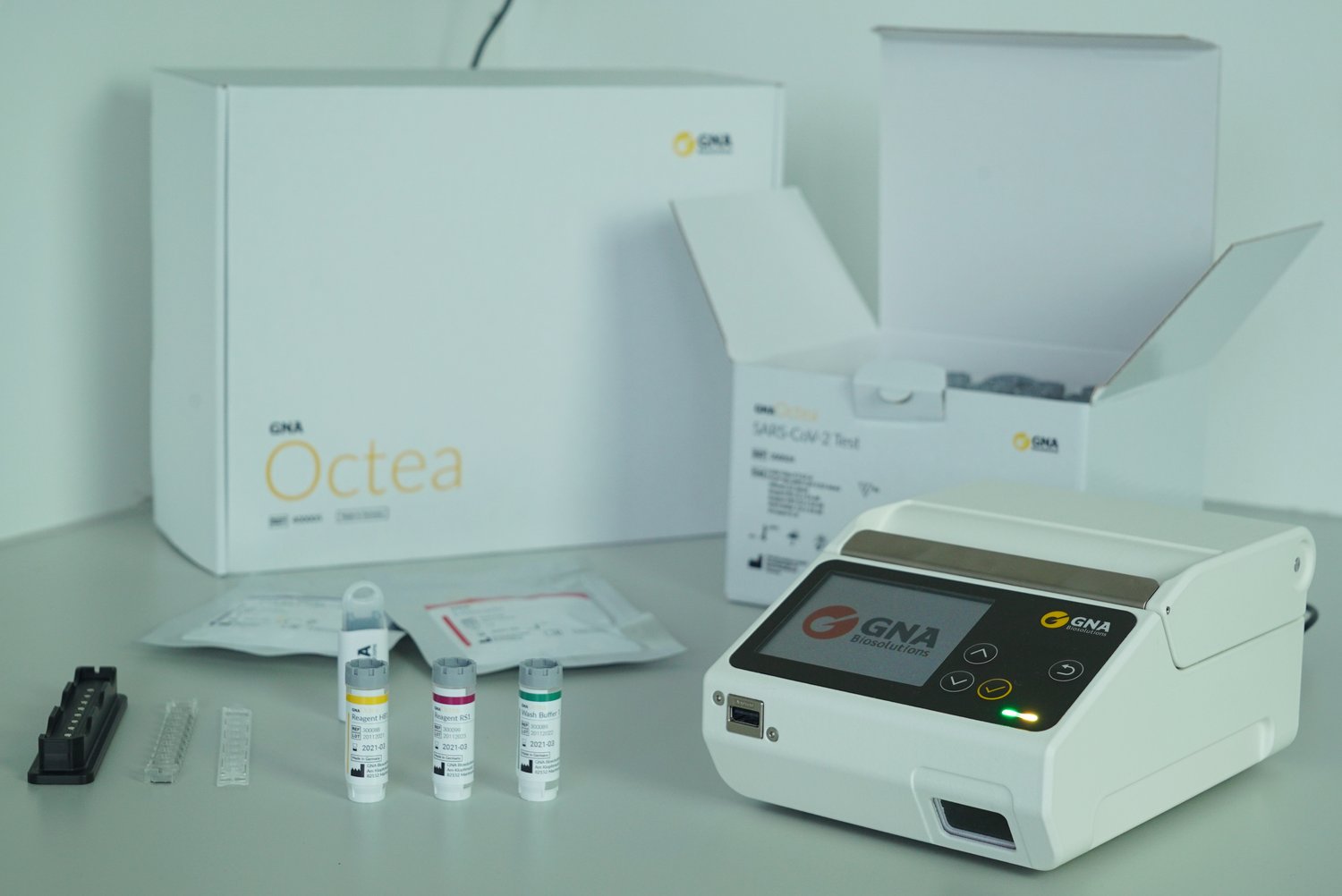
With a sensitivity of 96.7 percent and a specificity of 100 percent, GNA Octea achieves the high test reliability of conventional but slow PCR tests. The system also combines the time advantages of already available but comparatively less reliable rapid antigen tests: Within 20 minutes, the device analyzes up to eight samples in parallel. The total time including the times for throat swabs and test preparation is around 40 minutes. Conventional PCR tests with analysis in the laboratory often require two or more days until the test result is available. In addition, GNA Octea offers another decisive advantage: the test device is small, light, and mobile, so it can be set up and used quickly and easily anywhere. In addition to stationary test stations and doctors' offices, it therefore also opens new possibilities for temporary use in companies, schools, care facilities, on construction sites or at cultural events.
The development of the system software
The system software of the device includes the control software (embedded software), which executes functionalities in the device, as well as operating software, via which users can operate the device. In order for software to be used in medical devices and laboratory equipment or as a stand-alone medical product (e.g. medical apps), it must be developed in accordance with legal requirements (MDR, IVDR, FDA Guidelines & Regulations, etc.). The prerequisite for this is a certified quality management system in accordance with ISO 13485, which infoteam has had for many years as an experienced development partner and distributor. With infoteam's own development process iSOP.medical, which meets all the requirements of IEC 62304, infoteam is one of the few service providers in Europe that can implement software development according to both its own and external customer processes.
Outlook
By the end of March 2021, GNA and infoteam plan to complete the standard-compliant documentation (which is normally done in parallel with software development) and thereby apply for regular approval as an In vitro diagnostic (IVD). A further development of the device is planned afterwards.
Further information can be found in our employee magazine on p. 12f. (as of October 2020; German only) or on our homepage on the topic of "Software according to MDR, IVDR and FDA".
Please also refer to the press release of Bayerisches Staatsministeriums für Wirtschaft, Landesentwicklung und Energie (Bavarian State Ministry of Economic Affairs, Regional Development and Energy) dated December 29, 2020.
Contact at infoteam Software Group:
Patrick Kraus
Marketing Communications Manager
patrick.kraus@infoteam.de and presse@infoteam.de
+49 9131 78 00-860
Contact at GNA Biosolutions GmbH:
Anastasia Liapis
VP, Strategic Marketing & Partnerships
liapis@gna-bio.com
+49 89 99 82 07 195
Image rights
The attached graphical material is released for publication.
- Building-02.jpg
© infoteam Software AG
Possible picture caption: infoteam Software Group supported the software development of the PCR rapid test GNA Octea - DSC01803.jpg, DSC02073.jpg, DSC02317.jpg
© GNA Biosolutions GmbH
Potential picture caption: The GNA Octea rapid PCR tester delivers test results with the high confidence of the PCR method within 20 minutes.
About infoteam Software Group
infoteam Software Group has been implementing unique software solutions for their customers in the industry, infrastructure, life science and public service markets for almost 40 years. Its core business comprises the partial and total development of control and embedded software, middleware and application software – agile, modern and in line with the latest security requirements. Special areas of expertise include normatively regulated software for use in medical and laboratory equipment (IVDR, MDR, FDA, ISO 13485, IEC 62304, etc.) as well as functionally safe software at the highest safety level (IEC 61508, EN 50128, etc.). The service portfolio is well rounded as a result of many years of experience in data analysis, AI and machine learning.
infoteam Software Group employs more than 300 people and has offices and subsidiaries in Germany, the Czech Republic, Switzerland and China. The parent company, infoteam Software AG, is headquartered in Bubenreuth near Erlangen, Germany.